
Cancer researchers say circulating tumor DNA may be an important tool in clinical development and an early indicator of treatment benefit.

Four stakeholders request clarification of parts of an FDA draft guidance on clinical pharmacology considerations for human radiolabeled mass balance ...

Two stakeholders request changes to an FDA draft guidance on risk management plans to mitigate the potential for drug shortages.

FDA posts a draft guidance entitled Hydrogen Peroxide-Based Contact Lens Care Products: Consumer Labeling Recommendations Premarket Notification (510(...

Federal Register notice: FDA grants a 10/17-19 hearing request to review and vote on whether agency approval for Covis Makena (hydroxyprogesterone cap...

Federal Register notice: FDA withdraws approval of 30 NDAs from multiple applicants after they notified the agency that the products are no longer mar...
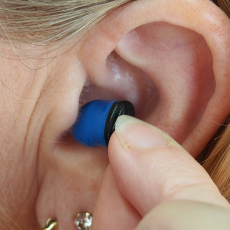
FDA authorizes personal sound amplification products as over-the-counter hearing aids for some consumers.

FDA publishes an updated guidance on replacement reagent and instrument family policy for in vitro diagnostic devices.