Pfizer and BioNTech file a submission seeking FDA Emergency Use Authorization for their booster dose of an Omicron BA.4/BA.5-adapted bivalent Covid-19...

After 38 years as National Institute of Allergy and Infectious Diseases (NIAID) director, Anthony Fauci says he will be stepping down at the end of th...

Federal Register notice: FDA seeks comments on an information collection extension entitled Medical Devices: Current Good Manufacturing Practice Quali...

Federal Register notice: FDA sends to OMB an information collection extension entitled Center for Devices and Radiological Health Appeals Processes.

A new research article by FDA and European regulators finds their good clinical practice inspection processes to be equivalent, which supports an ongo...
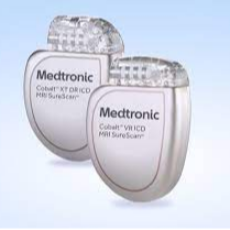
FDA classifies as Class 1 a recent Medtronic recall of its Cobalt/Crome implantable cardioverter defibrillators and resynchronization therapy defibril...

A new FDA report on Covid-19 activities notes that the agency is initiating a new inspection planning system that will be more efficient, transparent,...

A just-completed FDA inspection of Sun Pharmaceutical Industries Punjab, India manufacturing facility leads to a six-item Form FDA-483 that cites GMP ...