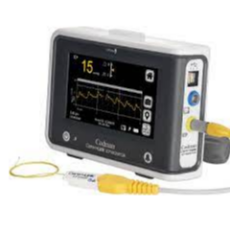
FDA says the Integra recall of 388 CereLink intracranial pressure monitors is Class 1.

Federal Register notice: FDA announces a 10/28 Oncologic Drugs Advisory Committee meeting.

Federal Register notice: FDA announces a 10/20 advisory committee meeting that will vote on a SolvD test to identify opioid use disorder at-risk patie...

FDA officials say the agency is taking a whole-of-governments approach to combating the entry of illicit healthcare products into the U.S.
FDA clears a Viz.ai algorithm to give the ratio between right and left ventricle diameters in a patients heart for use in pulmonary embolism treatment...

A Florida federal judge sentenced the former CEO of Pharmatech to 37 months in federal prison after he pled guilty to several charges of lying to and ...

Ascendis Pharma files an NDA for TransCon PTH, an investigational prodrug designed to restore parathyroid hormone (PTH [1-34]) to physiological levels...
FDA authorizes Modernas and Pfizer-BioNTechs updated (bivalent) Covid-19 vaccines for use as a single booster dose at least two months following prima...