
The Washington Legal Foundation says the 2nd Circuit Court of Appeals should affirm a lower courts dismissal of a suit against Forest Laboratories bec...
Federal Register notice: FDA issues a final rule specifying the deadline and content for submission of annual summaries under the Right to Try Act of...

Vanda Pharmaceuticals files a lawsuit against FDA seeking an order to compel it to publish in the Federal Register a notice of opportunity for a heari...
FDA releases a form FDA-483 with three observations from an inspection at an Aurobindo active pharmaceutical ingredient manufacturing facility in Andh...

FDA clears a Conformis 510(k) for its Acter Hip System to add a tri-taper femoral stem design to the device portfolio.
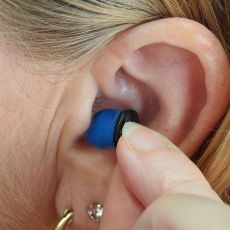
FDA denies a petition asking it to standardize hearing aid features in new over-the-counter hearing aids and rate them.

FDA joins the National Institutes of Health in launching a Critical Path for Rare Neurodegenerative Diseases public-private partnership.

Federal Register notice: FDA determines for patent extension purposes the regulatory review period for Eli Lillys migraine drug Reyvow (lasmiditan).