
Federal Register Notice: FDA announces an 11/1 Advisory Committee meeting to discuss ongoing concerns that pulse oximeters may be less accurate in cer...

FDA associate commissioner Mark Abdoo calls for greater collaborative efforts worldwide to combat illicit trade in healthcare products.
FDA says Baxter Healthcares recall of its Clearlink Basic Solution Sets with Duovent is Class 1.

Federal Register notice: FDA determines for patent extension purposes the regulatory review period for Intra-Cellular Therapies Caplyta.
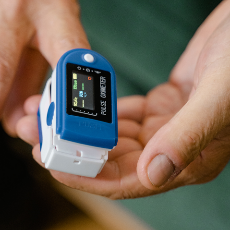
FDA says the Anesthesiology and Respiratory Therapy Devices Panel will meet virtually on 11/1 to discuss issues of potential pulse oximeter inaccuracy...

FDA publishes an International Conference on Harmonization revised guidance on elemental impurities in drug products.

Federal Register notice: FDA determines for patent extension purposes the regulatory review period for Rigel Pharmaceuticals Tavalisse.

CDER Office of Manufacturing Quality director Francis Godwin warns that FDA investigators are paying close attention to pharmaceutical water systems d...