
FDA determines there are insufficient data to support a proposed cefazolin susceptible minimum inhibitory concentration breakpoint of less than or equ...

FDA closes out a Warning Letter issued to Innova Medical Group over its unauthorized Covid-19 test following a recent inspection that did not cited an...

CDER Division of Hematologic Malignancies 2 division director Nicole Gormley is named acting associate director for oncology endpoint development in F...
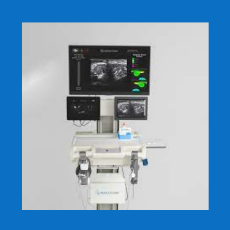
FDA grants Intelligent Ultrasound a de novo marketing authorization for its artificial intelligence ScanNav Anatomy Peripheral Nerve Block for ultraso...

Federal Register notice: FDA sends to OMB a proposed information collection entitled Annual Summary Reporting Requirements Under the Right to Try Act....

For the second time in a year, FDA inspects contract manufacturer Catalents Brussels, Belgium, manufacturing facility.

Federal Register notice: FDA extends the comment period for a 6/28 proposed rule entitled Nonprescription Drug Product With an Additional Condition fo...

Federal Register notice: FDA makes available a draft guidance entitled Select Updates for the Breakthrough Devices Program Guidance: Reducing Disparit...