
Representatives Kathy Castor (D-FL), Brian Fitzpatrick (R-PA), and Lauren Underwood (D-IL) introduce HR 9487, Advancing Safe Medications for Moms and ...

A Brookings Institution report suggests ways to speed FDA therapeutic equivalence ratings for injectable drugs approved through the 505(b)(2) process.

Harvard Medical School researchers call for new incentives to develop antibiotics that show added value for patients through clinical outcomes in pati...
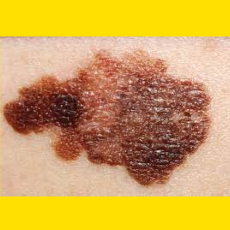
Moderna and Merck say their investigational mRNA cancer vaccine met its endpoint in a Phase 2b melanoma trial.

FDA clears a Bausch + Lomb 510(k) for its Biotrue Hydration Boost Contact Lens Rehydrating drops.

The Physicians Committee for Responsible Medicine calls on FDA to investigate possible good laboratory practice violations by Elon Musks Neuralink in ...

Federal Register notice: FDA sends to OMB an information collection extension entitled Medical Devices: Current Good Manufacturing Practice Quality Sy...

Smiths Medical sends an urgent medical device correction letter identifying two potential issues with CADD Infusion System Infusion Sets.