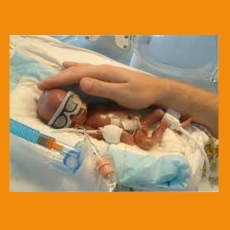
FDA says it is evaluating reports of exposure to airborne chemicals that may be associated with the use of neonatal incubators.

Four Democrat senators and representatives say the U.S. Patent and Trademark Office should weigh a new Merck patent request for a subcutaneous formula...
FDA releases the form FDA-483 issued following an inspection at the Ahmedabad, India-based Amneal Pharmaceuticals Private Limited parenteral manufactu...

CBER director Peter Marks discusses four lessons learned from the rapid development of Covid-19 vaccines and the implications for the future.

Federal Register notice: FDA determines that Allergans Asacol HD (mesalamine) delayed-release tablet, 800 mg, was not withdrawn due to safety or effec...
Federal Register notice: FDA determined that Janssens Topamax (topiramate) sprinkle capsules, 50 mg, was not withdrawn due to safety or effectiveness ...

The Association for Accessible Medicines praises the FDA revision of a draft guidance on generic drug controlled correspondence to meet new GDUFA 3 ag...
FDA publishes three guidances on aspects of x-ray system regulation.