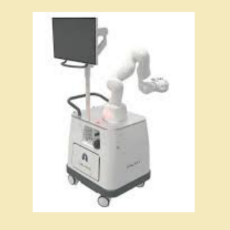
FDA clears a Noah Medical 510(k) for the Galaxy System, a robotic-navigated bronchoscopy device and accessories that are intended to provide bronchosc...
The CDER Office of Generic Drugs annual report says it approved 914 new ANDAs in 2022 and took many other steps under the GDUFA 3 reauthorization.

FDA publishes the International Council on Harmonization Q13 guidance on continuous manufacturing of drug substances and products.
FDA outlines new potential GMP issues under policy consideration in a discussion paper entitled Artificial Intelligence (AI) in Drug Manufacturing.

A jury returns a $43 million verdict against Precision Lens for violations of the False Claims Act and Anti-Kickback Statute.

FDA warns Ponce, Puerto Rico-based Skyless LLC about CGMP, new drug, misbranding, and other violations in its production of drug products.

FDA publishes a draft guidance with recommendations on assessing the potency of monoclonal antibodies and some other therapeutic proteins.

CDER associate director for rare diseases Kerry Jo Lee marks Rare Disease Day 2023 by highlighting the new Accelerate Rare disease Cures program.