
Three congressional Democrats introduce legislation to require drug companies to accurately account for how much they spend on drug research and devel...
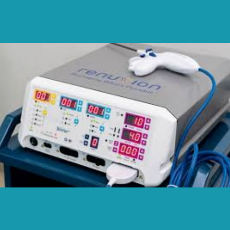
FDA clears the Apyx Renuvion/J-Plasma handpiece for coagulation of subcutaneous soft tissues following liposuction for aesthetic body contouring.
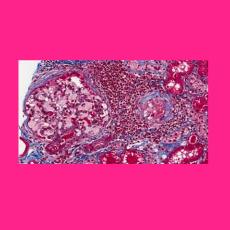
FDA approves a Chiesi Global Rare Diseases and Protalix BioTherapeutics BLA for Elfabrio (pegunigalsidase alfa-iwxj) for treating adult patients with ...

An FDA advisory committee recommends the approval of a Laboratoire HRA Pharma NDA seeking a prescription-to-nonprescription switch of its Opill, a nor...

FDA clears a Hubly Surgical 510(k) for the Hubly Drill and its use in performing burr hole procedures as part of brain surgery.
FDA announces a joint project with the National Institute of Standards and Technology to develop resources needed by animal biotechnology developers.

Federal Register notice: FDA determines for patent term extension purposes the regulatory review period for Somscans Detectnet (copper Cu-64 dotatate...

FDA publishes an immediately-effective guidance to help drug manufacturers, repackers, and compounders prevent the use of glycol-contaminated glycerin...