
Phoenix, AZ-based Pharmedica USA tells FDA it will stop all drug production and distribution after the agency finds CGMP violations during an inspecti...

Federal Register notice: FDA determines for patent term extension purposes the regulatory review period for Intact Vasculars Tack Endovascular System....

FDA releases the form FDA-483 with three observations issued following an April inspection at the Ipca Laboratories drug manufacturing facility in Hav...

FDA warns Waxahachie, TX-based Sea-Long Medical Systems about multiple Quality System regulation violations in its production of misbranded and adulte...

The Bespoke Gene Therapy Consortium, of which FDA is a member, chooses eight rare diseases for a clinical trial portfolio intended to speed the develo...

The Alliance for Pharmacy Compounding calls for changes to federal law to allow compounding pharmacies to help address temporary drug shortages under ...
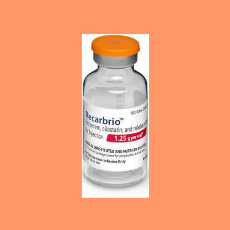
A BMJ investigation questions how FDA approved Mercks antibiotic Recarbrio in 2019 since it did not appear to meet agency requirements for substantial...

Federal Register notice: FDA determines for patent term extension purposes the regulatory review period for Cipla USAs Zemdri (plazomicin).