
The Senate Appropriations Committee approves FDAs appropriations bill, which provides the agency a $20 million spending increase.
FDA sends Intercept Pharmaceuticals a second complete response letter on its NDA resubmission for obeticholic acid for treating pre-cirrhotic fibrosis...

FDA posts a final guidance entitled Alternative Procedures for the Manufacture of Cold-Stored Platelets Intended for the Treatment of Active Bleeding ...

Federal Register notice: FDA releases a draft guidance entitled Formal Dispute Resolution and Administrative Hearings of Final Administrative Orders U...

Jazz Pharmaceuticals sues FDA and seeks the reversal of a 5/1 approval of Avadel CNS Pharmaceuticals Lumryz, a sodium oxybate drug product citing Jazz...
Centers for Medicare & Medicaid Services (CMS) issues a proposed procedural notice outlining a new Medicare coverage pathway for breakthrough medical ...
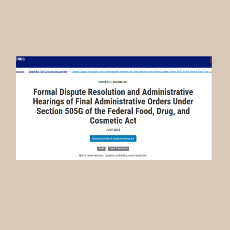
FDA publishes a draft guidance on the formal dispute resolution and administrative hearing process for over-the-counter monograph drugs.

Federal Register notice: FDA announces the renewal of the Medical Imaging Drugs Advisory Committee for an additional two years beyond the charter expi...