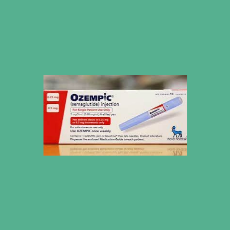
CBS News says a drug supply chain association issued an alert that FDA is investigating counterfeit Ozempic.

CDER says its NextGen Portal is now accepting Over-the-Counter Monograph Order Requests.

Coherus BioSciences resubmits a BLA supplement for Udenyca Onbody, the company's on-body injector presentation of Udenyca (pegfilgrastim-cbqv), a bios...

FDAs Oncologic Drugs Advisory Committee votes 10 to 2 that the CodeBreak 200 confirmatory study cannot be reliably interpreted as the basis for Amgens...

AstraZeneca suggests several things FDA could add to a draft guidance on QTc information in drug and biologic labeling.

The Reagan-Udall Foundation publishes a report with strategies for FDA to consider to improve public understanding of FDA and its regulated products.

CDRH director Jeffrey Shuren says FDA believes there is more Philips Respironics needs to do as part of its recall of ventilators and BiPAP and CPAP m...

FDA publishes a draft guidance to assist sponsors in developing drugs and biologics to treat stimulant use disorders.