
FDA grants Darmiyan a de novo marketing authorization for its clinical test BrainSee for assessing Alzheimers dementia risk.

Fresenius Kabi recalls its Ivenix Large Volume Pump because some units have mechanical issues with the fluid valve pins in the devices internal housin...

FDA approves Mercks Keytruda (pembrolizumab) with chemoradiotherapy (CRT) for treating patients with FIGO 2014 Stage III-IVA cervical cancer.

The Government Accountability Office says its testing of prenatal dietary supplements found that many had more or less nutrients than listed on the la...

FDA accepts for review a Shorla Oncology NDA for SH-105 for treating breast and ovarian cancer.

Canadian officials say they will act to prevent Floridas importation of drugs from that country to cause a shortage in Canada.

FDA publishes an ICH guidance on evaluating the viral safety of biotechnology products derived from human or animal cell lines.
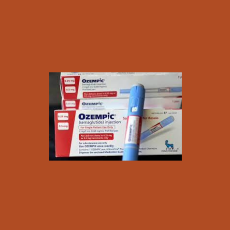
FDA says it has not been able to establish a causal connection between suicidal ideation and drugs like Ozempic and Wegovy and is continuing to review...