
FDA asks the Oncologic Drugs Advisory Committee to consider whether minimal residual disease is an appropriate intermediate clinical endpoint to suppo...

A new National Academies report calls on FDA and other federal government entities to take steps to increase the participation of pregnant and lactati...
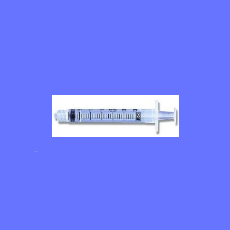
In the latest move by FDA in its ongoing investigation into the quality of syringes made in China, the agency issues an import alert against Jiangsu S...

Two stakeholders comment positively on an FDA public meeting on advancing the use of complex innovative designs in clinical trials.

CDER Office of Prescription Drug Promotion researchers say disclosing methodological rigor in communications to healthcare providers may help them for...
An FDA inspection last month at Alkem Laboratories India manufacturing facility leads to a 10-observation Form FDA-483.

A federal court approves an FDA-brokered consent decree against Philips Respironics over its defective breathing devices.

FDA warns Mumbai, India-based Kilitch Healthcare India about CGMP violations in its production of sterile ophthalmic drug products.