
FDA accepts for priority review an AstraZeneca supplemental BLA for Imfinzi (durvalumab) and its use in certain patients with limited-stage small cell...

FDA grants Wave Life Sciences a rare pediatric disease designation for WVE-N531 and its use in treating boys with Duchenne muscular dystrophy.

FDA approves a Vericel supplemental BLA for NexoBrid (anacaulase-bcdb) for eschar removal in pediatric patients with deep partial-thickness or full-th...
Two stakeholders respond to an FDA request for comments on the agencys recent listening session on advisory committees and their governing processes.

Federal Register notice: FDA seeks comments on the abuse potential, medical usefulness, trafficking, and impact of scheduling changes for eight drug s...

Federal Register notice: FDA announces a 10/7 Science Board Advisory Committee meeting that will hear an update from the new Alternative Methods Subco...
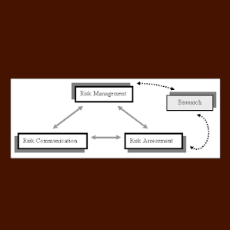
Two stakeholders call on FDA to make major changes to a draft guidance on the REMS logic model that is intended to link program design with assessment...

The drug development consulting firm Rahaa asks FDA to require stability studies under refrigerated conditions for generic versions of prednisolone op...