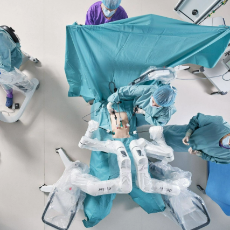
FDA grants Distalmotion a de novo marketing authorization for the Dexter Surgical Robot for adult inguinal hernia repair.
FDA clears a Paragonix Technologies 510(k) for its transportable perfusion device KidneyVault and its use in kidney preservation.

House Republicans demand the release of clinical data from taxpayer-funded research involving a study of puberty-blocking drugs that were not publishe...

Federal Register notice: FDA seeks comments on an information collection extension entitled Antimicrobial Animal Drug Sales and Distribution21 CFR 514...

A bipartisan Congressional effort seeks broader FDA interpretations when awarding priority review vouchers.

FarmaKeio Outsourcing, a drug compounding outfit, files a lawsuit against FDA seeking a court order to permit the compounding of sodium thiosulfate.
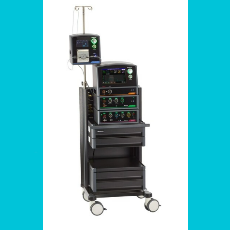
FDA approves Medtronics Affera Mapping and Ablation System with Sphere-9 Catheter for treating persistent atrial fibrillation.