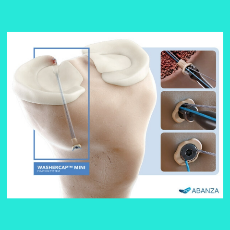
FDA clears an Abanza 510(k) for its WasherCap Mini fixation system for soft tissue repair, including meniscal root repair and anterior cruciate ligame...

FDA accepts for review a Regeneron Pharmaceuticals BLA resubmission for odronextamab in relapsed/refractory follicular lymphoma.

GenBioPro, which manufactures FDA-approved generic mifepristone, says it wants to be added to the list of defendants in a suit seeking to ban the drug...

Fresh on the heels of staff disruptions caused by the termination of about 1,000 probationary employees, the Trump administration issues a new memo ca...

FDA warns San Diego, CA-based Fertility Center of California about deviations from regulations governing cells, tissues, and cellular and tissue-based...

FDA accepts for priority review a Merck supplemental BLA for Keytruda (pembrolizumab) for treating patients with resectable locally advanced head and ...

FDA removes a required risk evaluation and mitigation strategies program for clozapine and its requirement to report results of absolute neutrophil co...
FDA releases the form FDA-483 issued following an inspection at the AstraZeneca Frederick, MD, drug manufacturing facility.